Serratia peptidase, or serrapeptase, is a proteolytic enzyme produced by the Serratia bacteria that lives in the intestines of silkworms. Serrapeptase has been used successfully for almost 40 years in Europe and Asia for its benefits in treating pain, inflammation, trauma-induced swelling and excessive mucous secretion, now available to buy in supplement form at SuperSmart.
What are the Benefits Associated to Serrapeptase?
Because serrapeptase is able to digest dead tissue – indeed, this is how it releases the silkworm from its cocoon – it can be used to restrict arterial obstruction (atheromatous plaques)and facilitate circulation. Hans Neiper, the world-renowned German doctor and scientist, has reported significant improvements from serrapeptase treatment in the circulation of a number of patients with previously compressed arteries. Serrapeptase can also:
- Act as an anti-inflammatory and analgesic, similar to aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs (NSAIDs).
- Induce fibrinolytic, anti-inflammatory, and anti-edema effects in many tissues; its anti-inflammatory effects are superior to those of other proteolytic enzymes. Serrapeptase works by fragmenting fibrinous aggregates, reducing the viscosity of exudates and by inhibiting the release of inflammatory mediators, thus facilitating drainage of these inflammatory response products thereby promoting the process of tissue repair.
- Help at reducing toxins in the intestine as well as reactions to foreign bodies.
- Boost antibiotic activity and tackle bacterial resistance by inhibiting the formation of their natural protective biofilm.
- It is particularly effective for relieving chronic ear, nose, and throat (ENT) inflammation, producing significant reductions in pain severity, quantity, and purulence of secretions, difficulty in swallowing, dysphonia, and nasal obstruction. Studies have highlighted its beneficial effects in normalising broncho-pulmonary secretions (mucous).
As with all enzymes, serrapeptase is sensitive to stomach acids. To improve its efficacy and bioavailability, new technology has enabled it to be produced in a gastro-resistant capsules, delayed release capsules, which release their content only in the small intestine.
Buy Serrapeptase capsules today to help fight inflammation.
WARNINGS
Do not exceed the recommended daily dose. This product is a nutritional supplement and should not be used as a substitute for a varied and balanced diet or a healthy lifestyle.
STORAGE
Store in a cool, dry place away from direct sunlight, heat, and humidity.
Keep out of reach of children.
PREGNANCY AND MEDICAL CONDITIONS
If you are pregnant, breastfeeding, or have any medical conditions, consult your healthcare provider before using this product.
SUPPLEMENT INTERACTIONS
Consult your healthcare provider before use, especially if you are taking any medications or other supplements as there may be potential interactions.
*These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.
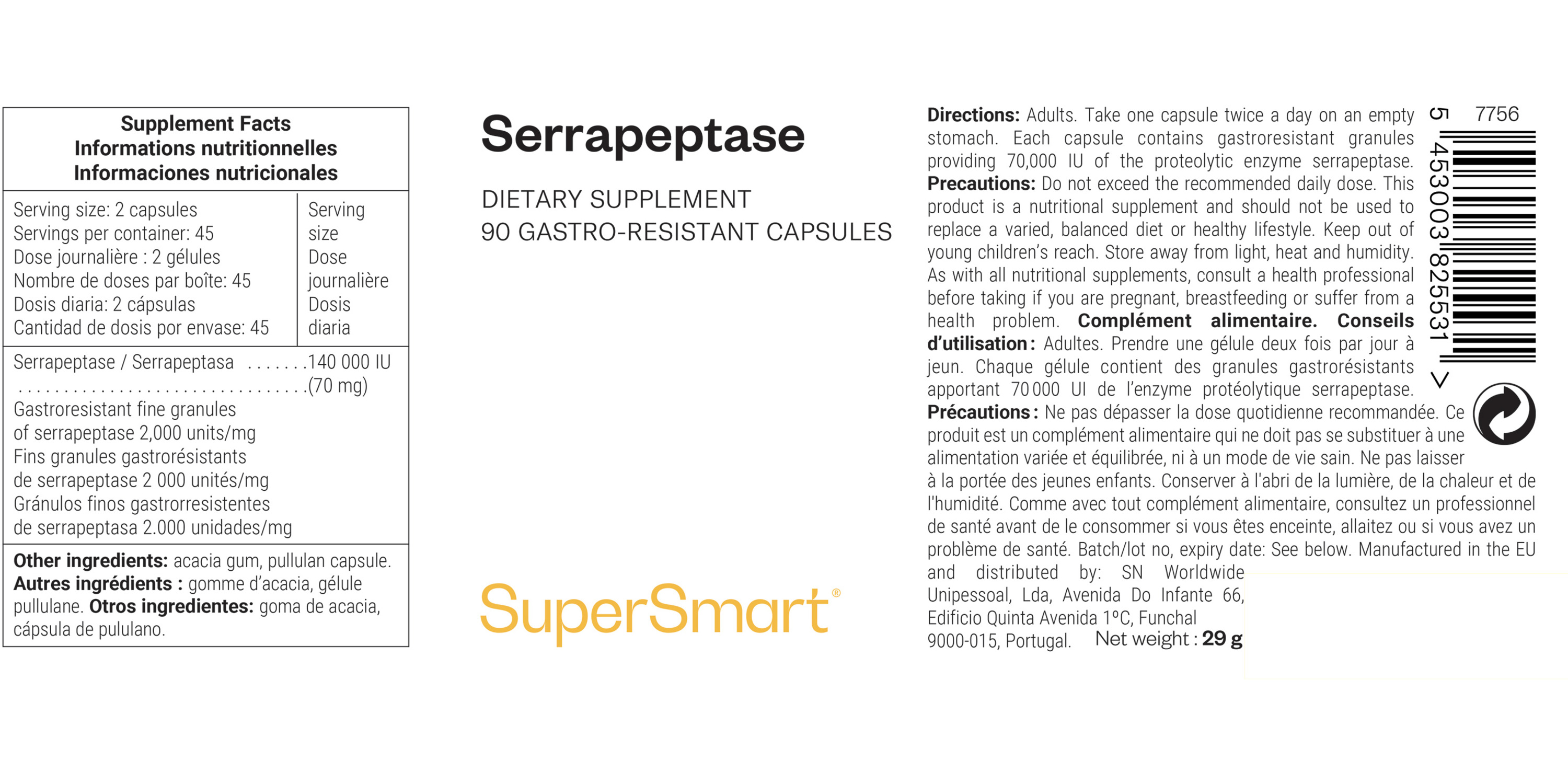
Serving Size: 2 Capsules
Servings Per Container: 45 |
Amount Per Serving |
Serrapeptase
Gastroresistant Fine Granules of Serrapeptase 2,000 units/mg |
140,000 IU (70 mg) |
|
Other Ingredients: Acacia Gum and Pullulan Capsule.
|
Serving size: Take 1 capsule twice a day on an empty stomach. Each capsule contains gastroresistant granules providing 70,000 IU of the proteolytic enzyme serrapeptase.
Servings per container: 45
Storage: Store away from direct light, heat, and humidity.
Warnings: Contains lactose.
Cautions: For adults only. Do not exceed the recommended daily dose. Keep out of reach of children. Consult a healthcare provider before taking this supplement and if you are pregnant, breastfeeding, or having health concerns.*
This product is a dietary supplement and should not be used as a substitute for a varied, balanced diet and a healthy lifestyle.
- Mazzone, M. Catalani, M. Costanzo, A. Drusian, S. Russo, et al.Evaluation of Serratia-peptidase in acute or chronic inflammation of otorhinolaryngology pathology: a multicentre, double-blind, randomized trial versus placebo, J Int Med Res, 18 (1990), pp. 379-388
- H. Yamasaki, H. Tsuji, K. Siki Anti-inflammatory action of a protease, TSP, produced by serratia, Folia Pharmacol Japan, 63 (1967), pp. 302-314
- G. Klein, W. Kullich, Short-term treatment of painful osteoarthritis of the knee with oral enzymes. A randomized, double-blind study versus diclofenac, Clin Drug Invest, 19 (2000), pp. 15-23
- Shivani Bhagat, MonikaAgarwal, Vandana Roy. Serratiopeptidase: A systematic review of the existing evidence, International Journal of Surgery, Volume 11, Issue 3, April 2013, Pages 209-217, https://doi.org/10.1016/j.ijsu.2013.01.010
- P. Libby, Inflammatory mechanisms: the molecular basis of inflammation and disease, Nutr Rev, 65 (12 Pt 2) (2007), pp. S140-S146
- C.N. Serhan, Novel chemical mediators in the resolution of inflammation: resolvins and protectins, Anesthesiol Clin, 24 (2) (2006), pp. 341-364
- G.L. Bannenberg, Resolvins: current understanding and future potential in the control of inflammation, Curr Opin Drug Discov Devel, 12 (5) (2009), pp. 644-658
- G. Klein, W. Kullich, Short-term treatment of painful osteoarthritis of the knee with oral enzymes. A randomized, double-blind study versus diclofenac, Clin Drug Invest, 19 (2000), pp. 15-23
- N. Moriya, A. Shoichi, H. Yoko, H. Fumio, K. Yoshiaki, Intestinal absorption of serrapeptase and its distribution to the inflammation sites, Japan Pharmacol Therap, 31 (2003), pp. 659-666
- N. Moriya, M. Nakata, M. Nakamuma, M. Takaoka, S. Iwasa, K. Kato, et al. Intestinal absorption of serrapeptase (TSP) in rats, Biotechnol Appl Biochem, 20 (1994), pp. 101-108
- S.P. Jadav, N.H. Patel, T.G. Shah, et al. Comparison of anti-inflammatory activity of serratiopeptidase and diclofenac in albino rats. J Pharmacol Pharmacother, 1 (2) (2010), pp. 116-117, 10.4103/0976-500X.72362
- M.M. Bodhankar, V.V. Agnihotri, S.B. Bhushan, Feasibility, formulation and characterization of innovative microparticles for oral delivery of peptide drug, Int J Res Pharm Chem, 1 (2011), pp. 630-636
- Bhagat S, et al., Serratiopeptidase: A systematic review of the existing evidence, International Journal of Surgery (2013), https://dx.doi.org/10.1016/j.ijsu.2013.01.010